Do you search for 'how to write sodium oxide'? All material can be found on this website.
What is the chemical formula of Sodium Oxide? The chemical chemical formula of Sodium Oxide is Na2O. IT is also noted as disodium oxide. ...In which applications sodium oxide is used? Sodium oxide is used for making glasses and ceramics. It is also used for making light-weight structures in aerospace and advanced electronics.What happens when hydrogen reacts with sodium oxide? Sodium Oxide absorbs hydrogen to make over sodium hydride and NaOH or Na hydroxide. ...Is atomic number 11 oxide toxic? The sodium oxide is a substance that is corrosive to eyes, respiratory tracts, and skin. Inhalant of the kernel may cause lung oedema. ...What ar the related chemic compounds of Na oxide?Appearance: Yellow Clear SolidCompound Formula: Na2OMelting Point: 1,132°C (2,070° F)Molecular Weight:
Table of contents
- How to write sodium oxide in 2021
- Magnesium oxide formula
- Sodium oxide cation
- Sodium oxide chemical structure
- Sodium hydroxide
- Sodium oxide + water
- Aluminum oxide formula
- Sodium oxide symbol
How to write sodium oxide in 2021
 This image demonstrates how to write sodium oxide.
This image demonstrates how to write sodium oxide.
Magnesium oxide formula
 This image shows Magnesium oxide formula.
This image shows Magnesium oxide formula.
Sodium oxide cation
 This picture illustrates Sodium oxide cation.
This picture illustrates Sodium oxide cation.
Sodium oxide chemical structure
 This image demonstrates Sodium oxide chemical structure.
This image demonstrates Sodium oxide chemical structure.
Sodium hydroxide
 This image shows Sodium hydroxide.
This image shows Sodium hydroxide.
Sodium oxide + water
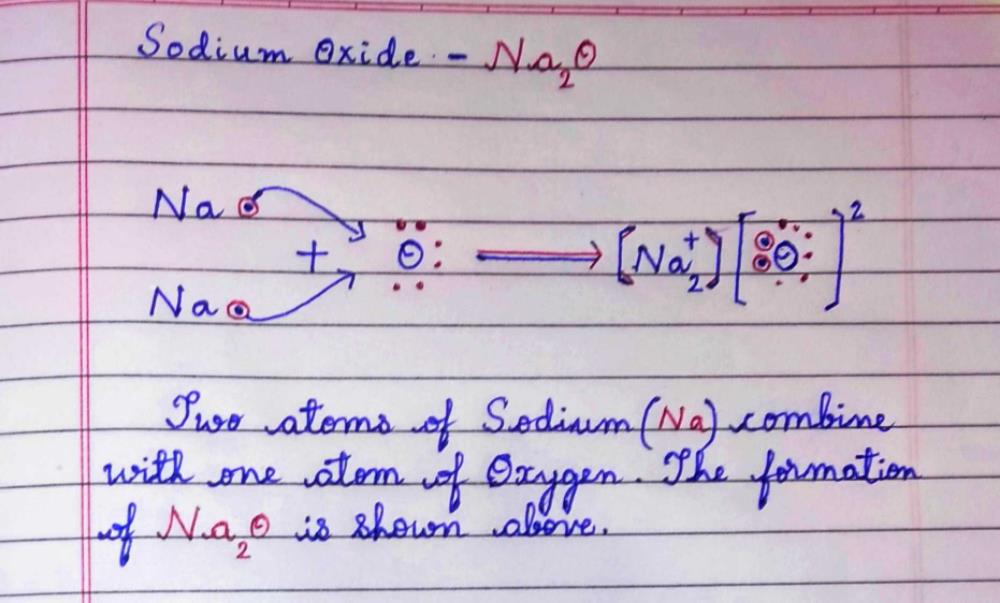 This picture representes Sodium oxide + water.
This picture representes Sodium oxide + water.
Aluminum oxide formula
 This picture demonstrates Aluminum oxide formula.
This picture demonstrates Aluminum oxide formula.
Sodium oxide symbol
 This picture demonstrates Sodium oxide symbol.
This picture demonstrates Sodium oxide symbol.
Which is the correct formula for sodium oxide?
Sodium oxide is a chemical compound with the formula Na 2 O. It is used in ceramics and glasses. The compound is the base anhydride of sodium hydroxide; when water is added to sodium oxide, NaOH is produced. Na 2 O + H 2 O → 2 NaOH. The alkali metal oxides M 2 O (M = Li, Na, K, Rb) crystallise in the antifluorite structure.
How is sodium oxide produced in the laboratory?
Burning sodium in air will produce Na 2 O and about 20% sodium peroxide Na 2 O 2 . A more accessible way of producing it in the laboratory consists in decomposing the sodium salt of ascorbic acid at temperatures over 209 Celsius degrees. Sodium oxide is a component of most glass, although it is added in the form of "soda" ( sodium carbonate ).
What's the cheapest way to make sodium oxide?
Need to produce >95% pure Sodium Oxide, and want to know the easiest/cheapiest way to make it. Does Sodium Carbonate decomposes completely at >900ºC (or 1000ºC, 1100ºC) to Sodium Oxide?
What is sodium oxide and what is it used for?
Sodium oxide is also used in the manufacture of commercial glasses based on the oxides of silicon and certain additives. To learn more about sodium oxide and other important chemical compounds containing sodium (such as sodium bicarbonate ), download BYJU’S – The Learning App.
Last Update: Oct 2021